Tutivia ™
A precise multi-gene RNA signature developed through AI techniques to provide an early warning blood-based assay that can detect all types of acute rejection, preceding traditional markers. Clinicians can use this test for both high risk or low risk individuals from the first week post-transplant and during follow-ups, regardless of delayed graft function, multiple transplants, or BK nephropathy.


Actionable Risk Scores for Every Patient
Visit our Expert's Corner page to explore two compelling case studies showcasing Tutivia's clinical utility–one on managing patients with delayed draft function and the other in managing patients with BK Virus.
Click here to explore.
VericiDx is excited to announce that our Tutivia validation study is available as open access in the March American Journal of Transplantation.
To access the Tutivia validation study click here.
Videos
Tutivia in Kidney Transplantation

Curious about RNA in biomarkers? Tune into our fire side chat led by transplant nephrologists discussing the role of biomarkers in transplant care and introducing the next generation of risk assessment - Tutivia.
-
Dr. Zahraa Hajjiri, MD
Medical Director of Transplant Nephrology,
University of Illinois Chicago -
Dr. M. Javeed Ansari, MD, MRCP
Associate Professor of Medicine, Division of Nephrology,
Associate Professor of Surgery, Division of Organ Transplantation,
Northwestern University Feinberg School of Medicine -
Prince Mohan Anand MD, FACP, FASN, FAST
Director of Transplant Services,
MUSC Mid-Carolinas
Coffee & Cases Episode 2: Clinical Management of BK
Coffee & Cases Episode 1: Delayed Graft Function
Personalized Testing for Individualized Care
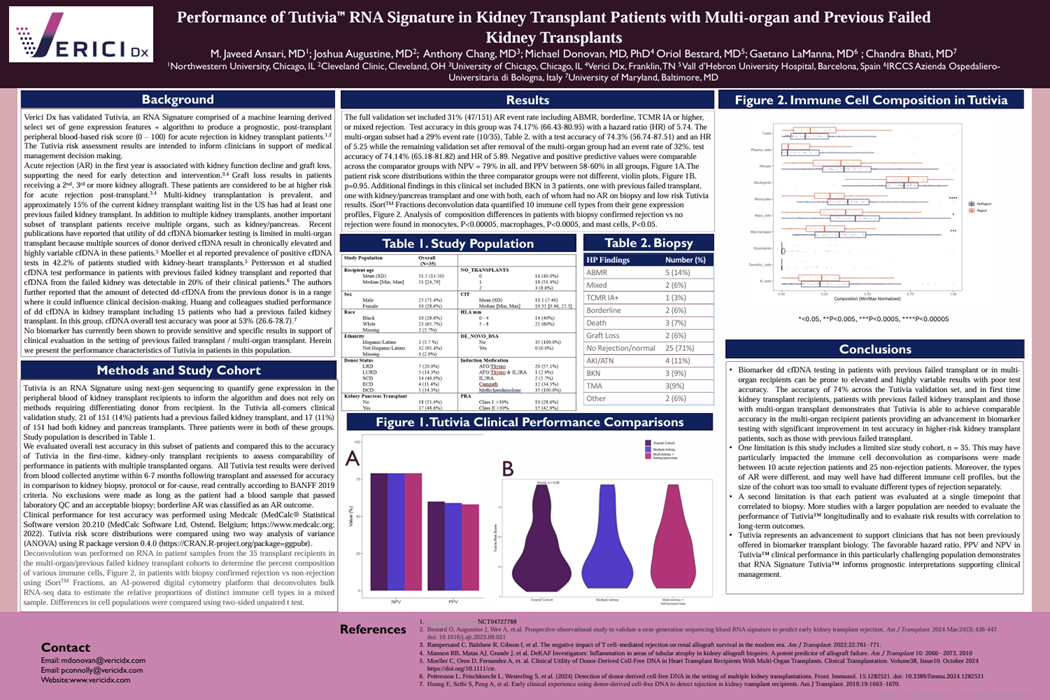
Posters & Papers